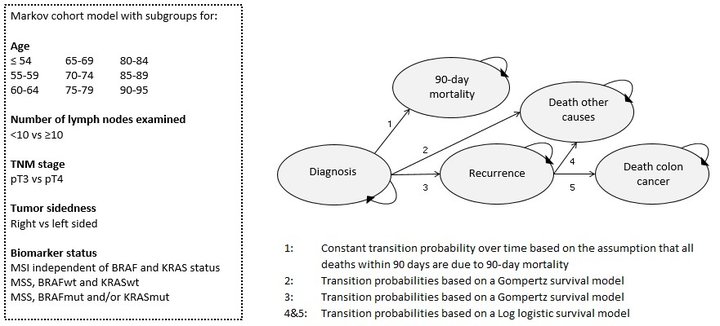
Therefore, we developed a Markov cohort model that can be used to evaluate strategies for the allocation of adjuvant chemotherapy in stage II colon cancer patients. Five health states were included in the Personalized Adjuvant TreaTment in EaRly stage coloN cancer (PATTERN) model; diagnosis, 90-day mortality, death other causes, recurrence and colon cancer death. A dataset from the Netherlands Cancer Registry (n=2,271) was used to parameterize the model. Transition probabilities were estimated using parametric survival models including relevant clinical and pathological covariates. Subsequently, biomarker status was implemented using data from three external clinical cohorts. Treatment effect was incorporated based on pooled data of 9 RCTs. The Figure below shows the flowchart of the PATTERN model.
Effectiveness and cost-effectiveness of the optimal treatment duration
Using the PATTERN decision model, we evaluated the efficacy of 3 versus 6 months of adjuvant treatment with either CAPOX or FOLFOX. In this study, we concluded that the optimal treatment duration for CAPOX is 3 months, given the higher health benefits and lower costs compared to a treatment duration of 6 months. These findings are in line with the recent adjustments to the Dutch guidelines, in which the recommended treatment duration for CAPOX has been reduced from 6 months to 3 months for stage II colon cancer patients. The study also showed that 6 months is the optimal treatment duration for FOLFOX, given the additional health benefits compared to a treatment duration of 3 months.
Effectiveness and cost-effectiveness of risk-based selection strategies
As there is no consensus on which high-risk stage II patients to select for adjuvant chemotherapy, we evaluated the effectiveness and cost-effectiveness of 5 risk-based selection strategies. We demonstrated that all evaluated strategies in which (part of the) patients were treated with adjuvant chemotherapy were more effective and costlier compared to a strategy in which none of the patients received adjuvant chemotherapy. Based on a willingness-to-pay of 50,000 €/QALY, current selection of stage II colon patients for chemotherapy in The Netherlands can be improved by either including biomarker status in the selection strategy or improving adherence to current Dutch guideline recommendations.
Furthermore, we evaluated the potential of including Consensus Molecular Subtypes (CMS) in the treatment decision for stage II colon cancer by conducting an early cost-effectiveness study. A CMS-based selection strategy was compared to the current Dutch guidelines as well as to a selection strategy based on MMR, BRAF and KRAS status. Based on the limited evidence available, the optimal use of resources would be to allocate adjuvant chemotherapy based on MMR, BRAF and KRAS status assuming a willingness-to-pay threshold of 50,000 €/QALY. However, a value of information analysis showed that the CMS, MMR, BRAF, and KRAS model parameters were the biggest drivers of uncertainty. Thus, additional research is needed to reduce decision uncertainty for these parameters.